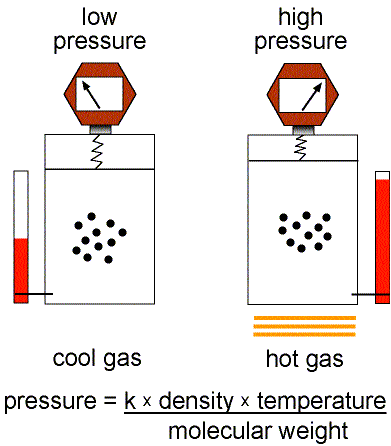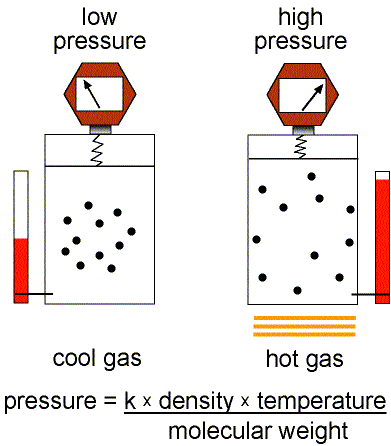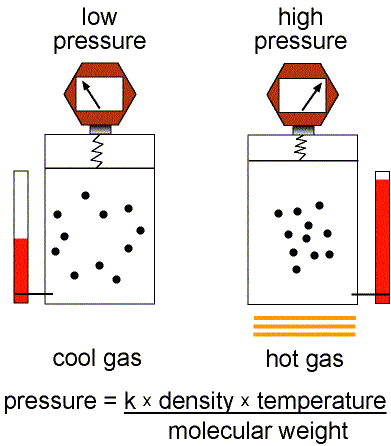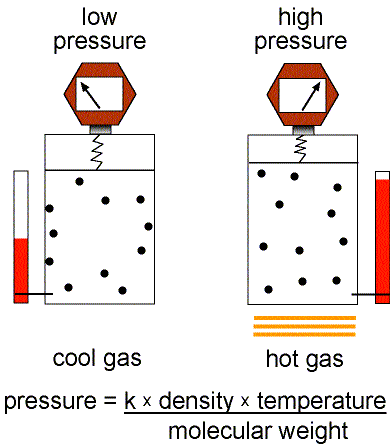
This equation of state for simple gases is also called the ideal gas law.
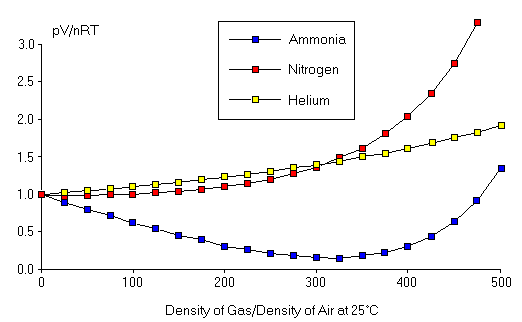
Figure 2: Deviations from the Ideal Gas Law.

the ideal gas law. Boyle's law describes the inversely

The cores of normal stars are an ideal gas, and follow the ideal gas law.

and the ideal gas law is used to find the amount of a certain gas.

Implications of Ideal Gas Law.

ideal gas law

Avogadro's Law: (V/n = constant) This is a direct relationship.

Macroscopic properties of matter are governed by the Ideal Gas Law of

The ideal gas law states that the volume taken up by a given number of

This is exactly the condition that we saw the molecules of an ideal gas had
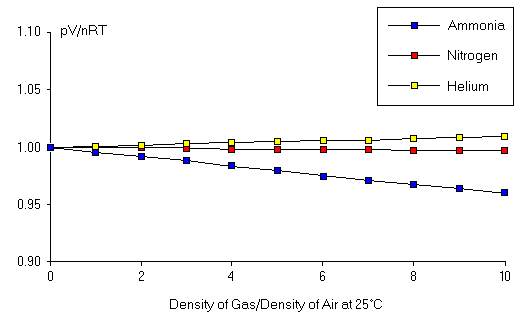
Figure 1: Validity of the Ideal Gas Law.

Ideal gas law

The ideal gas law asserts that when two other variables are held constant:

Ideal Gas Law - Assumptions

Deviations from the Ideal Gas Law (fig2.png)
Ideal gas law: PV=nRT. Thus, temperature is proportional to (1/2) mv2.
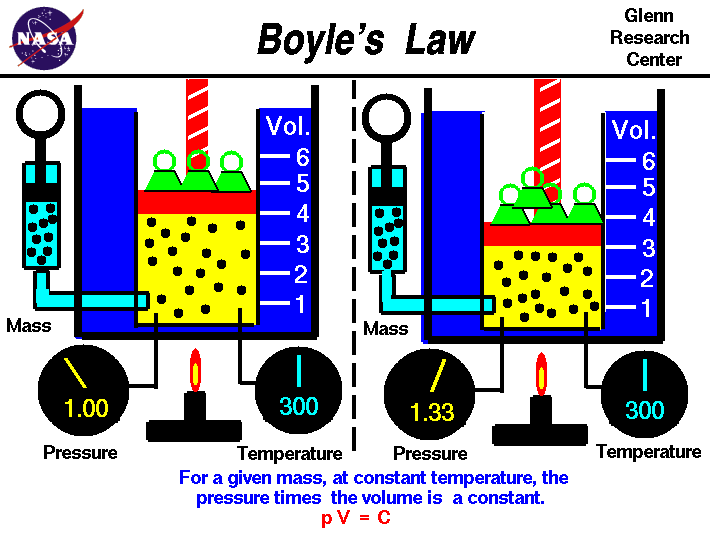
Boyle's law relates the pressure and volume of an ideal gas.

Charles and Gay-Lussac's law relates the temperature and volume of an ideal

Visit Images Browser for Daily Updated Hairstyles Collection








